In Japan, when a medical device recall occurs, there is an obligation to immediately publicize it.
The PMDA (Pharmaceuticals and Medical Devices Agency), an agency affiliated with the MHLW (Ministry of Health, Labor and Welfare), is in charge of public announcements.
New Arrivals List
ACL TOP 300 CTS CTS
Blood Coagulation Analyzer
IL Japan K.K.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12341
MeKiKi Database [2-12341]
Inspired Flow
Heated Humidifier Inspired Medical Japan
Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12342
MeKiKi Database [2-12342]
Hugo RAS System
Surgical Robotic Surgical Unit
Covidien Japan Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12348
MeKiKi Database [2-12348]
SCD Smart Flow
Sequential Pneumatic Massager
Cardinal Health Inc.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12349
MeKiKi Database [2-12349]
SCD Smart Flow
Intermittent pneumatic massager
Cardinal Health Inc.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12349
MeKiKi Database [2-12349]
Extend Tip Applicator
Infusion catheter
Integra Japan Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12350
MeKiKi Database [2-12350]
CT900 Programmer for
Physicians Programmer for Implantable Active Devices
Japan Medtronic Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12351
MeKiKi Database [2-12351]
CT900 Programmer for
Physicians Implantable Active Device Management Program
Japan Medtronic Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12351
MeKiKi Database [2-12351]
Micro knife
single-use scalpel
Kai Industries Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12352
MeKiKi Database [2-12352]
Microblading
Single-Use Scalpel Blade
Kai Industries Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12352
MeKiKi Database [2-12352]
CGA Pressure Reducer High
Pressure Gas Regulator Wako
Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12353
MeKiKi Database [2-12353]
Guidewire (sterile)
Single-use bone surgery instrument Japan MDM
Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12355
MeKiKi Database [2-12355]
Baylis Transceptal Sheath
Cardiac Catheter Introducer Kit
Boston Scientific Japan K.K.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12356
MeKiKi Database [2-12356]
Baylis RF Transceptal Wire
Active lancing device for transseptum
Boston Scientific Japan K.K.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12356
MeKiKi Database [2-12356]
Radiation Therapy Planning Software RayStation
Radiation Therapy Planning Program
RaySearch Japan K.K.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12357
MeKiKi Database [2-12357]
multipurpose imaging system VersiFlex VISTA
Stationary Digital General-Purpose X-ray Fluoroscopy System
FUJIFILM Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12360
MeKiKi Database [2-12360]
JINS One Day Colored Contact Lenses
for
Single-Use Vision Correction PEGAVISION JAPAN Co., Ltd
.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12361
MeKiKi Database [2-12361]
Intersurgical i-view
Video rigid intubation laryngoscope
MC Medical Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12363
MeKiKi Database [2-12363]
Bardex Silverlubrisil Temperature sensor catheter
Antimicrobial urological catheter Medicon
Inc.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12364
MeKiKi Database [2-12364]
BD Insight AutoGuard
Plastic Cannula Sterile Puncture Needle
Japan Becton Dickinson Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12366
MeKiKi Database [2-12366]
CareLink SmartSync Device Manager
Programmer for Implantable Active Devices
Japan Medtronic Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12367
MeKiKi Database [2-12367]
fully automated hematology analyzer PCE-350
Hematology Analyzer
Eruma Sales Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12368
MeKiKi Database [2-12368]
Proton Beam Therapy System PROBEAT-RT
Particle Beam Therapy System Hitachi,
Ltd. Medical Device Division
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12370
MeKiKi Database [2-12370]
LSO1470 Laser
Diode Laser Medicos Hirata
Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12372
MeKiKiKi Database [2-12372]
Silicon Seal
Endoscope Component Adapter
Olympus Medical Systems Corporation Hachioji Plant
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12375
MeKiKi Database [2-12375]
Two-Me/Eric Evacurator
Temporary use bladder lavage kit
Olympus Medical Systems Corporation Hachioji Plant
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12375
MeKiKi Database [2-12375]
Cancellous Bone Structure Indicator Software
Programs for
bone X-ray absorptiometers Toyo Medic Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12376
MeKiKi Database [2-12376]
Lasen Entrance Tracheostomy Tube
Reinforced tracheostomy tube
for ventilation Fuji Systems Co., Ltd. Shirakawa Factory
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12380
MeKiKi Database [2-12380]
DexCom G7 CGM System
Glucose Monitor System
DexCom Japan G.K.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12381
MeKiKi Database [2-12381]
AARON900 Foot switch for electrosurgical instrument components
Reusable Hand Control Electrosurgical Electrode
Gunze Medical Co., Ltd. Tokyo Branch
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12382
MeKiKi Database [2-12382]
Intraosseous Pharmaceutical Injection Device NIO
Single-use intraosseous injection needle Wako
Shoji
Co., Ltd. https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12383
MeKiKi Database [2-12383]
digital general radiography system RADspeed safire Stationary
digital general-purpose X-ray diagnostic system Shimadzu
Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12384
MeKiKi Database [2-12384]
X-Ray Television System SONIALVISION G4
Stationary Digital General-purpose X-ray Fluoroscopy System Shimadzu
Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12384
MeKiKi Database [2-12384]
angiography system Trinias
Stationary digital cardiovascular X-ray fluoroscopy system Shimadzu
Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12384
MeKiKi Database [2-12384]
radiotherapy device motion tracking system SyncTraX
Synchronizer for radiotherapy equipment Shimadzu
Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12384
MeKiKi Database [2-12384]
diagnostic X-ray high-voltage system UD150B-40
Stationary diagnostic X-ray generator Shimadzu
Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12384
MeKiKi Database [2-12384]
RILINE Spinal System
Intravertebral Fixation Device
New Basement Japan Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=3-2988
MeKiKi Database [3-2988]
OMRON Portable Electrocardiograph HCG-8060T
Seizure Cardiac Activity Recording Device
OMRON Healthcare Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=3-2989
MeKiKi Database [3-2989]
SIOS FIT
Mobile Digital General-purpose Integrated X-ray Fluoroscopy System
Siemens Healthcare Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=3-2990
MeKiKi Database [3-2990]
Arcatena Disposable Kit
Angiography Kit Nemoto Kyorindo
Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=3-2993
MeKiKi Database [3-2993]
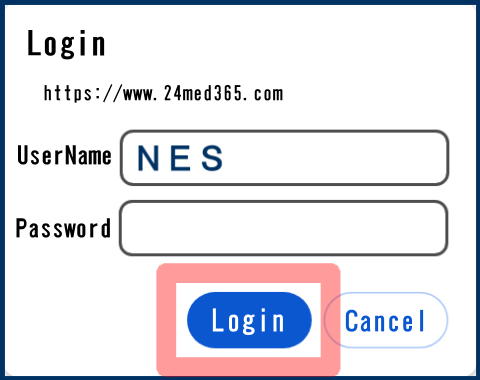
When viewing the database, a login screen may pop up as shown in the figure below. This is a measure to avoid excessive load caused by robot searches. We appreciate your cooperation.
Please enter “NES” as your user name to login.