In Japan, when a medical device recall occurs, there is an obligation to immediately publicize it.
The PMDA (Pharmaceuticals and Medical Devices Agency), an agency affiliated with the MHLW (Ministry of Health, Labor and Welfare), is in charge of public announcements.
New Arrivals List
Hospirite LEDX
Surgical Lighting System
Daiichi Lighting Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12464
MeKiKi Database[2-12464]
Jet Washer Cleaning System MU-6200V
Instrument Decontamination Cleaning Machine
Sharp Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12466
MeKiKi Database[2-12466]
SANAPDO Sutures
Polydioxanone Sutures
Genesis Medtech Japan K.K.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12468
MeKiKi Database[2-12468]
Electrocardiograph (with Analysis Function)MAC VU360
General Purpose Electrocardiograph
GE Healthcare Japan K.K. https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12470
MeKiKi Database[2-12470]
MAGNETOM Vida
Superconducting Magnet Type Whole Body MR Equipment
Siemens Healthcare Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12473
MeKiKi Database[2-12473]
Biograph mMR
Combined Positron CT Equipment
Siemens Healthcare Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12473
MeKiKi Database[2-12473] Catalyst
Catalyst System
Synchronizer for Radiation Therapy Equipment
Electa Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12475
MeKiKi Database[2-12475]
VIM-NT
Orthodontic Wire
Oral Care Co., Ltd. https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12476
MeKiKi Database[2-12476]
Dexcom G6 CGM System
Glucose Monitor System
Cowbridge Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12477 MeKiKi Database[2-12477]
SafeTech Pro Syringe Pump Unit
Syringe Infusion Pump
Nipro Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12478
MeKiKi Database[2-12478]
Cure Fit Accessories Blood
Transfusion & Catheter Accessory Set
JMS Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12479
MeKiKi Database[2-12479]
Cure Fit Accessories
Accessory Set for Infusion and Catheter
JMS Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12479 MeKiKi Database[2-12479]
Cure Fit Extension Tube
Extension Tube for Blood Transfusion and Catheter
JMS Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12479
MeKiKi Database[2-12479]
Cure Fit Extension Tube
Extension Tube for Infusion Pump
JMS Co., Ltd
. https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12479
MeKiKi Database[2-12479]
Bird Silver TSC Tray
Antibacterial Urinary Catheter
Medicon Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12480 MeKiKi Database[2-12480]
Bird I.C. Foley Tray B
Short-term use urinary Foley catheter
Medicon Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12480
MeKiKi Database[2-12480]
CRS-Master Treatment Planning
OphthalmologySurgical Treatment Planning Program
Carl Zeiss Meditech Inc.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12489
MeKiKi Database[2-12489]
Proton Beam Therapy System,
Particle Beam Therapy Equipment
Sumitomo Heavy Industries, Ltd., Ehime Works, Niihama Plant
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12490
MeKiKi Database[2-12490]
Mediloop
Surgical Tape
Medicos Hirata Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12491
MeKiKi Database[2-12491]
Gyroscan Intra 1.5T
Superconducting Magnet Type Whole Body MR Equipment
Philips Japan Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12495
MeKiKi Database[2-12495]
Port X IV
Digital extraoral general-purpose dental X-ray diagnostic equipment Morita Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12496
MeKiKi database[2-12496]
Port X IV
Analog Extraoral General Purpose Dental X-ray Diagnostic System
Morita Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12496
MeKiKi Database[2-12496]
PortX IV
Digital extraoral general-purpose dental X-ray diagnostic equipment Morita Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12497
MeKiKi database[2-12497]
Port X IV
Analog Extraoral General Purpose Dental X-ray Diagnostic System
Morita Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12497
MeKiKi Database[2-12497]
IMPELLA Controller
Circulatory Support Intracardiac Indwelling Pump Catheter Control System
Japan Aviomed Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12498
MeKiKi Database[2-12498]
Automatic Thermal Jade Chiropractor (SIATSU)
Home Heated Shiatsu Substitute
Shen Pex International Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12499
MeKiKi Database[2-12499]
Rounding Radiography System MobileDaRt Evolution
Mobile Digital General-Purpose X-ray Diagnostic System
Shimadzu Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12500
MeKiKi Database[2-12500]
CADD Anesthesia Infusion Set
Anesthesia Infusion Set
ICU Medical Japan K.K.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12504
MeKiKi Database[2-12504]
Fully Automatic Blood Transfusion Test System Erytra
Blood typing analyzer
Kainos Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12505
MeKiKi database[2-12505]
Hugo RAS Instrument
Reusable Endoscopic Active Procedure Device
Covidien Japan K.K.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12506
MeKiKi Database[2-12506]
CARESCAPE Central Station
Central monitor with analysis
GE Healthcare Japan K.K. https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12507
MeKiKi Database[2-12507]
Portable Inhaler Misty
Home UltrasoundInhaler
A&Day Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12508
MeKiKi Database[2-12508]
Signia Small Diameter Reload Tissue Staple
for Internal Fixation
Covidien Japan K.K.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12510
MeKiKi Database[2-12510]
Laccept Blade Introducer
Cardiac Catheter Introducer Kit
Goodman Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12511
MeKiKi Database[2-12511]
BIOLOX delta Ceramic Head (CERAMAX) Hip
Prosthesis Femoral Component
Johnson & Johnson Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=3-3012
MeKiKi Database[3-3012]
Acreget FLY
Single-use intraocular lens inserter
Wakamoto Pharmaceutical Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=3-3015
MeKiKi database[3-3015]
Acriva Torinova Pro
Multifocal Posterior Chamber Lens
Wakamoto Pharmaceutical Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=3-3015
MeKiKi Database [3-3015]
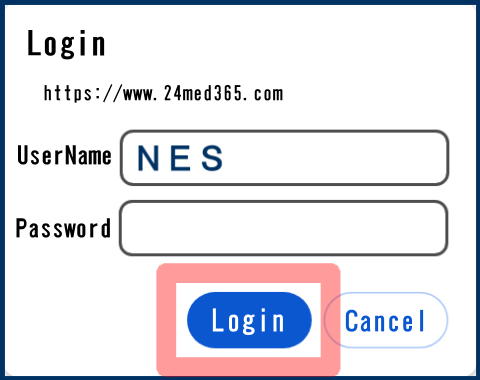
When viewing the database, a login screen may pop up as shown in the figure below. This is a measure to avoid excessive load caused by robot searches. We appreciate your cooperation.
Please enter “NES” as your user name to login.