In Japan, when a medical device recall occurs, there is an obligation to immediately publicize it.
The PMDA (Pharmaceuticals and Medical Devices Agency), an agency affiliated with the MHLW (Ministry of Health, Labor and Welfare), is in charge of public announcements.
New Arrivals List
Friarit 2 Abutment
Dental Implant Abutment
Dents Ply Sirona Co., Ltd
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12594
NES Medical Database[2-12594]
Sophisa Presio ICP Monitor
Intracranial Pressure Monitor
TKB
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12595
NES Medical Database[2-12595]
CT900 Physician Programmer
Implantable Active Device Management Program
Japan Medtronic Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12596
NES Medical Database[2-12596]
Celastland-SL
Radiation Source for Manual Brachtherapy Equipment for Permanent Penetration of Non-central Circulatory System
Japan Meji Physics Co., Ltd
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12597
NES Medical Database[2-12597]
Cerastrand-SL
Polyglactin
Sutures Japan Mediphyx Co.,Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12597
NES Medical Database[2-12597]
QT Access Catheter
Single-use endoscopic inactive procedure device for artificial orifice
Machida Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12598
NES Medical Database[2-12598]
Air Dermatome
Gas Pressure Dermatome
Zimmer Biomet LLC
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12600
NES Medical Database[2-12600]
Medtronic Vanta PC
Implantable Pain Relief Stimulator
Japan Medtronic Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12601
NES Medical Database[2-12601]
bf-Sensor Sheet I Type
Dental Occlusal Force Meter
Sumitomo Riko Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12602
NES Medical Database[2-12602]
Tabletop Endoscope Cleaning and Disinfecting Instrument EFEWD
Flexible Endoscope Cleaning and Disinfecting Device
Dai-ichi Medical Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12603
NES Medical Database[2-12603]
Hoya One Delight
Reusable Vision Correction Colored Contact Lenses
From Eyes Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12604
NES Medical Database[2-12604]
Sure Fuser A
Pressurized Pharmaceutical Injector
Nipro Co., Ltd
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12605
NES Medical Database[2-12605]
Latitude Programming System 3300
Programmer for Implanted Active Equipment
Boston Scientific Japan K.K.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12606
NES Medical Database[2-12606]
CT900 Physician Programmer
Implantable Active Device Management Program
Japan Medtronic Corporation
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12608
NES Medical Database[2-12608]
Hickman Catheter Kit
Central Venous Catheter Introducer Kit
Medicon Co.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12609
Ltd. NES Medical Database[2-12609]
Elekta Unity MR Linear Accelerator System
Linear Accelerator System
Elekta Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12612
NES Medical Database[2-12612]
da Vinci SP Access Port Kit
Single-Use Pioneer
Intuitive Surgical LLC
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12613
NES Medical Database[2-12613]
Quanta Cyber Blade Motorator
Endoscopic Active Resection Instrument
EDAP Technomed Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12614
NES Medical Database[2-12614]
Carestation 600 Series
Anesthesia System
GE Healthcare Japan K.K.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12615
NES Medical Database[2-12615]
Hot AXIOS System
Pancreatic Fistula Formation Drainage Stent
Boston Scientific Japan K.K.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12616
NES Medical Database[2-12616]
Audiometer AA-77A
Pure Tone Audiometer
Rion Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12618
NES Medical Database[2-12618]
CODMAN MicroSensor Basic Kit
Catheter with Transducer for Intracranial Pressure Measurement
Integra Japan Co., Ltd
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12619
NES Medical Database[2-12619]
IMRIS HFD100 Head Fixation Device
Head Surgery Clamp
Saikyo Biotech Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=2-12622
NES Medical Database[2-12622]
Hologic Fluent In/Out-FloPak Tube Set
Water Pump Suction Tube
Hologic Japan Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=3-3033
NES Medical Database[3-3033]
PHILIPS Disposable ElectrodesSingle-Use
ECG Electrodes
Philips Japan Co., Ltd
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=3-3034
NES Medical Database[3-3034]
IJ Tape
Emergency Adhesive Bandage
Piac Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=3-3035
NES Medical Database[3-3035]
contrast media injection system PRESSPRO GEO-150
multiphase electric contrast media injection system
Nemoto Kyorindo Co., Ltd. https://www.info.pmda.go.jp/rgo/MainServlet?recallno=3-3036
NES Medical Database[3-3036]
Hot Dog Patient Warming System
Electric Pad Warming Device Control Unit
Muranaka Medical Equipment Co., Ltd
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=3-3037
NES Medical Database[3-3037]
Hot Dog Patient Warming SystemElectric
Pad Warming SystemMuranaka
Medical Equipment Co., Ltd.
https://www.info.pmda.go.jp/rgo/MainServlet?recallno=3-3037
NES Medical Database[3-3037]
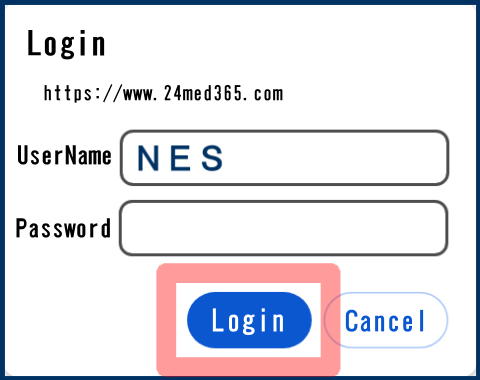
When viewing the database, a login screen may pop up as shown in the figure below. This is a measure to avoid excessive load caused by robot searches. We appreciate your cooperation.
Please enter “NES” as your user name to login.